Our work focusses on the biology of the central and peripheral emotion circuits, brain basis of social bonding and social cognition, neuroscience of musical emotions, and neuromolecular basis of feeding and metabolic disorders. In addition to high-resolution total-positron emission tomography, we use multimodal neuroimaging with functional and structural MRI as well as behavioural recording techniques such as eye tracking. This page showcases or main research projects and lists key readings for each topic. Our work on signal analysis and the software packages released by Emotion Lab are listed on the resources page.
Mapping total-body emotion circuits

Emotions guide humans in constantly changing dynamic environments. In addition to providing rapid fight-or-flight responses during survival-salient encounters, emotions are also social signals that are expressed to other individuals, which provides important information for adjusting social interaction with that individual. We use state-of-the art total-body positron emission tomography (PET) and functional neuroimaging and behavioural and psychophsyiological measurements for mapping the whole-body circuits governing emotional responses and their expressions in health and disease. Additionally, we are investigating the link between emotion and somatosensation to reveal how emotions are represented not only in the mind, but also in the body. We also use PET for quantifying the role of the specific neuromolecular pathways in emotions related to anxiety, attachment, feeding, and sexuality. This allows us to reveal novel pharmacological targets for disorders involving deficits in social and motivated behaviour.
Key papers on emotions
1. Nummenmaa, L., Hari, R., Hietanen, J.K., & Glerean, E. (2018). Maps of subjective feelings. PNAS, 115, 9198–9203.
2. Nummenmaa, L., Glerean, E., Viinikainen, M., Jääskeläinen, I.P., Hari, R., & Sams, M. (2012) Emotions promote social interaction by synchronizing brain activity across individuals. PNAS, 109, 9599–9604.
3. Nummenmaa, L., Glerean, E., Hari, R., & Hietanen, J.K. (2014). Bodily maps of emotions. PNAS, 111, 646-651.
4. Knuuti, J., Tuisku, J., Kärpijoki, H., Iida, H., Maaniitty, T., Latva-Rasku, A., Oikonen, V., Nesterov, S., Teuho, J., Jaakkola, M., Klen, R., Louhi, H., Saunavaara, V., Nuutila, P., Saraste, A., Rinne, J., & Nummenmaa, L. (2023). Quantitative perfusion imaging with total body positron emission tomography. Journal of Nuclear Medicine, 64, 11-19.
Brain basis of social cognition

Humans have long-lasting affiliative relationships in all areas of life. These bonds persist despite of infrequent physical contact. Thus, they must be supported by a system that is decoupled from sexual response and direct physical contact. Using PET, fMRI and behavioural recordings we study the contribution of different molecular pathways – dopaminergic, opioidercid and serotonergic systems – on social bonding and affiliation. We also investigate the mechanism that enable coordinated activity across groups of individuals. Understanding other peoples’ minds is one of the most fundamental human skills but also one of the most demanding challenges our brains must pose every day: To understand each others’ actions and intentions, we need to share internal neural representations of the external world across brains. Recent advances in the brain signal analysis enable us to study the foundations of brain basis of human social interaction under highly naturalistic settings with unparalleled accuracy and ecological validity. By mapping the brain basis of natural social vision using rich and detailed stimulus models we can bridge the gat between classic social psychology and cognitive neuroscience by building a comprehensive neurocognitive model of how individuals maintain and communicate shared neural representations of the dynamic social world.
Key papers on social cognition
1. Suvilehto, J., Glerean, E., Dunbar, R.I.M., Hari, R., & Nummenmaa, L. (2015). Topography of social touching depends on emotional bonds between humans. PNAS, 115, 13811-13816.
2. Manninen, S., Tuominen, L., Dunbar, R.I.M., Karjalainen, T., Hirvonen, J., Arponen, E., Hari, R., Jääskeläinen, I.P., Sams, M., & Nummenmaa, L. (2017). Social laughter triggers endogenous opioid release in humans. The Journal of Neuroscience, 37, 6125-6131.
3. Santavirta, S., Malen, T., Erdemli, A., &, Nummenmaa, L. (2024). A taxonomy for human social perception: Data-driven modeling with cinematic stimuli. Journal of Personality and Social Psychology, 127, 1146-1171.
4. Nummenmaa, L., & Calder, A.J. (2009). Neural mechanisms of social attention.Trends in Cognitive Sciences, 13(3), 135-143.
Neuroscience of musical emotions
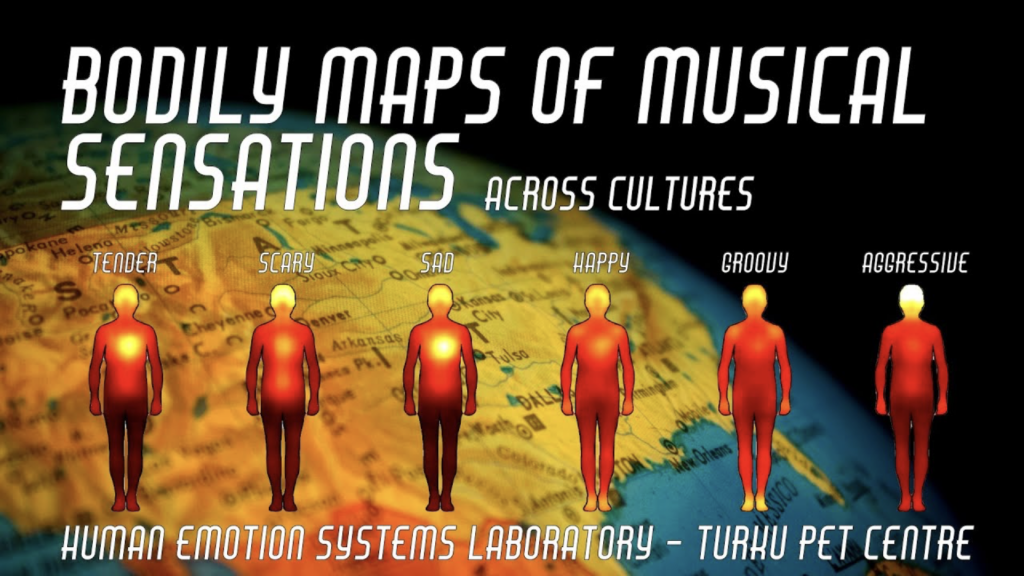
Music is central to our emotional life. Humans across all societies engage in music-listening and making, which they find pleasurable. Music is however a curious delight. Emotional responses to music show many hallmarks of emotions in general including distinct subjective experience, emotional expression, action tendencies, changes in the autonomic nervous system activation and distinct effects on thought and judgement, despite the fact that music itself serves no obvious adaptive value. However, many of the affective functions may arise from the social nature of music, and the strong links between music, interpersonal synchrony and affiliation are important factors that make music so rewarding. Musical autobiographical memories and social and emotional lyrics in music further provide means for rehearsing and maintaining various social scripts. Because of their episodic nature, music-evoked autobiographical memories are oftentimes imbued with feeling of nostalgia. In this research project we use both behavioural measures as well as state-of-the-art positron emission tomography to map the central and peripheral basis of musical emotions as well as the functions of the musical affects.
Key papers on musical emotions
1. Putkinen, V., Seppälä, K., Harju, H., Hirvoinen, J., Karlsson, H.K., & Nummenmaa, L. (2025). Pleasurable music activates cerebral μ-opioid receptors: A combined PET-fMRI study. European Journal of Nuclear Medicine and Molecular Imaging.
2. Putkinen, V., Zhou, X., Gan, X., Yang, L., Becker, B., Sams, M., & Nummenmaa, L. (2024). Bodily maps of musical sensations across cultures. PNAS, 121, e2308859121.
3. Putkinen, V., Nazari-Farsani, S., Seppälä, K., Karjalainen, T. Sun, L., Karlsson, H., Hudson, M., Heikkilä, T.K., Hirvonen, J., & Nummenmaa, L. (2021). Decoding music-evoked emotions in the auditory and motor cortex. Cerebral Cortex, 31, 2549-2560.
4. Nummenmaa, L., Putkinen, V., & Sams, M. (2021). Social pleasures of music. Current Opinion in Behavioral Sciences, 39, 196-202.
Neuromolecular basis of obesity and eating disorders
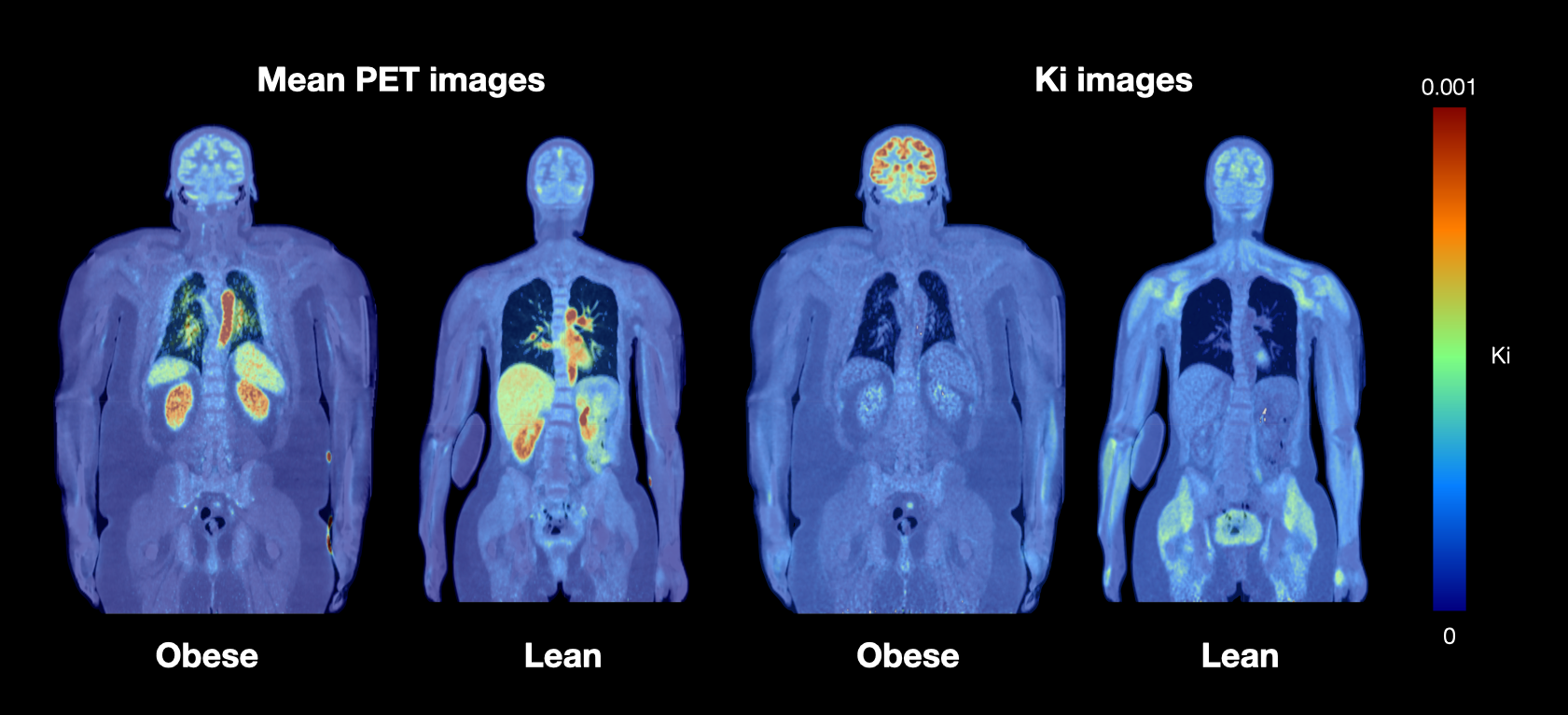
Diet and nutrition are critical factors in the maintenance of good health throughout the entire life course. Their role as determinants of chronic diseases such as obesity, diabetes and cardiovascular diseases is well established. However, the annual increase in the prevalence and the severity of obesity in both adults and children is currently substantial. Phenomenological similarities between obesity and addictive behaviors have led researchers to suggest that addictions may provide a framework for obesity. The most clearly established commonality of the mechanisms of drug and food intake is their ability to activate the dopamine containing link in the brain’s reward systems. Although exaggerated sensitivity to high-calorie food cues may be a critical factor explaining obesity, the exact neural basis of the individual differences in food-induced reward and subsequent eating habits are not fully understood. Our multimodal PET – fMRI studies in healthy and obese participants as well as those with eating disorders are aimed at determining how the tonic and phasic activity of the reward system predict self-reported eating habits and being both over- and underweight. The functional data are complemented by structural MRI data, tractographic analyses of diffusion weighted data, and neurotransmitter PET studies.
Key papers on obesity and eating disorders
1. Pak, K., Tuisku, J., Karlsson, H.K., Hirvonen, J., Rebelos, E., Pekkarinen, L., Sun, L., Latva-Rasku, A., Helin, S., Rajander, J., Karukivi, M., Nuutila, P., & Nummenmaa, L. (2024). Anorexia nervosa is associated with higher brain mu-opioid receptor availability. Molecular Psychiatry.
2. Karlsson, H.K., Tuulari, J.J., Tuominen, L., Hirvonen, J., Honka, H., Parkkola, R., Helin, S., Salminen, P., Nuutila, P., & Nummenmaa, L. (2016). Weight loss after bariatric surgery normalizes brain opioid receptors in morbid obesity. Molecular Psychiatry, 21, 1057-1062.
3. Nummenmaa, L., Saanijoki, T., Tuominen, L., Hirvonen, J., Tuulari, J.J., Nuutila, P., & Kalliokoski, K (2018). μ-opioid receptor system mediates reward processing in humans. Nature Communications, 9, 1-7.
4. Laurila, S., Lahesmaa, M., Sun, L., Brain, K., Laitinen, K., Niemi, T., Taittonen, M., Din, M.U., Klen, R., Välikangas, T., Elo, L., Eskola, O., Kirjavainen, A., Nummenmaa, L., Virtanen, K.A., Klingenspor, M., & Nuutila, P (2021). Secretin activates brown fat and induces satiation in humans. Nature Metabolism, 3, 798-809.
PET Methods Development
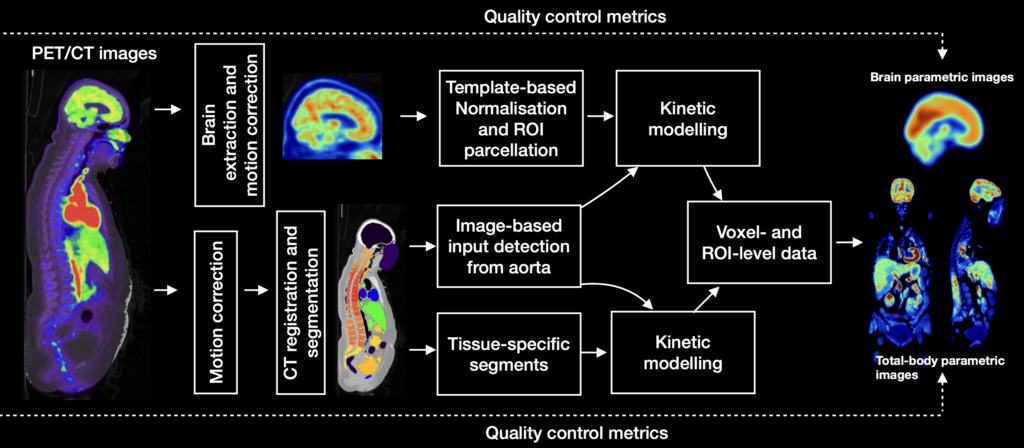
Processing of positron emission tomography (PET) data from single-organ studies (such as brain) data involves intensive manual work, causing inter-operator variance and poor replicability. These issues are mitigated in long axial field of view (Total-body) PET imaging where the large number of target tissues increases manual workload, necessitating a high level of automatization and standardization. Our PET methods development is focussing on developing fully automated pipelines incorporating motion correction, registration, input extraction and modelling, region-of-interest parcellation and kinetic modelling. The MAGIA toolbox is dedicated for brain-only studies whereas the TURBO toolbox is a general-purpose solution for total-body studies and incorporates MAGIA functions for brain analysis. Both toolboxes are open-source and freely available and under active development.
Key papers on PET Methods
1. Tuisku, J., Palonen, S., Kärpijoki, H., Latva-Rasku, A., Tuomola, N., Harju, H., Nesterov, S., Oikonen, V., Iida, H., Teuho, J., Han, C., Karjalainen, T., Kirjavainen, A.K., Rajander, J., Klen, R., Nuutila, P., Knuuti, J., & Nummenmaa, L. (2026). Automated Total-body PET Image Processing and Kinetic Modeling with the TURBO Toolbox. European Journal of Nuclear Medicine and Molecular Imaging.
2. Karjalainen, T., Tuisku, J., Santavirta, S., Kantonen, T., Bucci, M., Tuominen, L., Hirvonen, J., Hietala, J., Rinne J., & Nummenmaa, L. (2020). Magia: Robust automated image processing and kinetic modeling toolbox for PET neuroinformatics. Frontiers in Neuroinformatics.